Polio was once a disease feared worldwide, striking suddenly and paralysing mainly children for life.
Polio now survives only among the world’s poorest and most marginalized communities, where it stalks the most vulnerable children.
The development of effective vaccines to prevent paralytic polio was one of the major medical breakthroughs of the 20th century. The Global Polio Eradication Initiative uses two types of vaccine to stop polio transmission – inactivated polio vaccine (IPV) and oral polio vaccine (OPV).
If enough people in a community are immunized against polio, the virus will be deprived of susceptible hosts and will die out. High levels of vaccination coverage must be maintained to stop transmission and prevent outbreaks occurring. The Global Polio Eradication Initiative is constantly assessing the optimal use of the different types of vaccine to prevent paralytic polio and stop poliovirus transmission in different areas of the world.
Oral poliovirus vaccine (OPV)
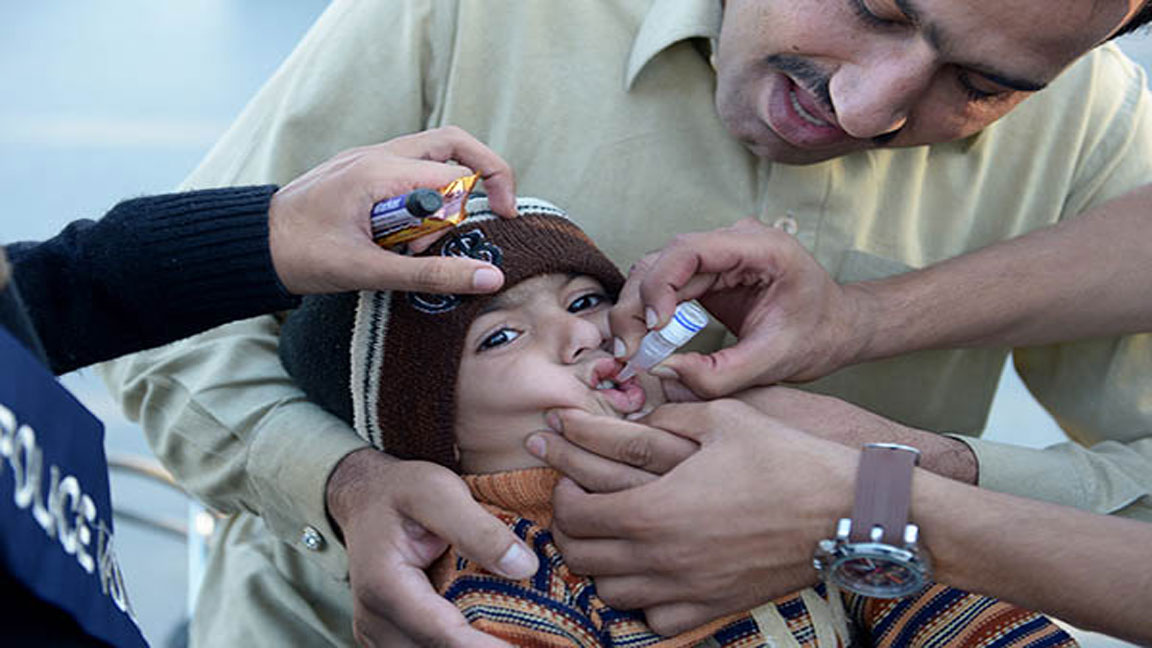
Oral poliovirus vaccines (OPV) are the predominant vaccine used in the fight to eradicate polio. There are different types of oral poliovirus vaccine, which may contain one, a combination of two, or all three different serotypes of attenuated vaccine. Each has their own advantages and disadvantages over the others.
The attenuated poliovirus(es) contained in OPV are able to replicate effectively in the intestine, but around 10,000 times less able to enter the central nervous system than the wild virus. This enables individuals to mount an immune response against the virus. Virtually all countries which have eradicated polio used OPV to interrupt person to person transmission of the virus.
Advantages
OPVs are all inexpensive (US $0.12-$0.18 for countries procuring through UNICEF in 2016).
OPVs are safe and effective and offer long lasting protection against the serotype(s) which they target. OPV stimulates good mucosal immunity, which is why it is so effective at interrupting transmission of the virus.
OPVs are administered orally and do not require health professionals or sterile needle syringes. As such,
OPVs are easy to administer in mass vaccination campaigns.
For several weeks after vaccination the vaccine virus replicates in the intestine, is excreted and can be spread to others in close contact. This means that in areas with poor hygiene and sanitation, immunization with OPV can result in ‘passive’ immunization of people who have not been vaccinated.
Disadvantages
OPV is extremely safe and effective. However, in extremely rare cases (at a rate of approximately 2 to 4 events per 1 million births [1]) the live attenuated vaccine-virus in OPV can cause paralysis. In some cases, it is believed that this may be triggered by an immunodeficiency.
The extremely low risk of vaccine-associated paralytic poliomyelitis (VAPP) is well accepted by most public health programmes.
Very rarely, when there is insufficient coverage in a community the vaccine-virus may be able to circulate, mutate and, over the course of 12 to 18 months, reacquire neurovirulence. This is known as a circulating vaccine-derived poliovirus.
Monovalent oral poliovirus vaccine (mOPV)
Prior to the development of tOPV, monovalent OPVs (mOPVs) were developed in the early 1950s, but largely dropped out of use upon the adoption of tOPV. It was not available at the time of the founding of
GPEI in 1988. Monovalent oral polio vaccines confer immunity to just one of the three serotypes of OPV. They are more successful in conferring immunity to the serotype targeted than tOPV, but do not provide protection to the other two types.
Monovalent OPVs for type 1 (mOPV1) and type 3 (mOPV3) poliovirus were licensed again in 2005, thanks to successful action taken by the GPEI. They elicit the best immune response against the serotype they target of all the vaccines.
Monovalent OPV type 2 (mOPV2) has been stockpiled in the event of a cVDPV2 outbreak.
Novel oral polio vaccine type 2 (nOPV2)
To better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2), GPEI partners are working to deploy an additional innovative tool – novel oral polio vaccine type 2 (nOPV2). more.
Bivalent oral poliovirus vaccine (bOPV)
Following April 2016, the trivalent oral poliovirus vaccine was replaced with the bivalent oral poliovirus vaccine (bOPV) in routine immunization around the world. Bivalent OPV contains only attenuated virus of serotypes 1 and 3, in the same number as in the trivalent vaccine.
Bivalent OPV elicits a better immune response against poliovirus types 1 and 3 than trivalent OPV, but does not give immunity against serotype 2. As well as in routine immunization, bOPV will be used for outbreak response against poliovirus types 1 and 3 outbreaks.
Trivalent oral poliovirus vaccine (tOPV)
Prior to April 2016, the trivalent oral poliovirus vaccine (tOPV) was the predominant vaccine used for routine immunization against poliovirus. Developed in the 1950s by Albert Sabin, tOPV consists of a mixture of live, attenuated polioviruses of all three serotypes. Also called the ‘Sabin vaccine’, tOPV is inexpensive and effective, and offers long lasting protection to all three serotypes of poliovirus.
The trivalent vaccine was withdrawn in April 2016 and replaced with the bivalent oral poliovirus vaccine (bOPV), which contains only attenuated virus of types 1 and 3. This is because continued use of tOPV threatened to continue seeding new type 2 circulating vaccine-derived polioviruses (cVDPV2), despite the wild type 2 virus being eradicated in 1999.
Inactivated poliovirus vaccine (IPV)
Inactivated polio vaccine (IPV) was developed in 1955 by Dr Jonas Salk. Also called the Salk vaccine IPV consists of inactivated (killed) poliovirus strains of all three poliovirus types. IPV is given by intramuscular or intradermal injection and needs to be administered by a trained health worker. IVP produces antibodies in the blood to all three types of poliovirus. In the event of infection, these antibodies prevent the spread of the virus to the central nervous system and protect against paralysis.
Advantages
As IPV is not a ‘live’ vaccine, it carries no risk of VAPP.
IPV triggers an excellent protective immune response in most people.
Disadvantages
IPV induces very low levels of immunity in the intestine. As a result, when a person immunized with IPV is infected with wild poliovirus, the virus can still multiply inside the intestines and be shed in the faeces, risking continued circulation.
IPV is over five times more expensive than OPV. Administering the vaccine requires trained health workers, as well as sterile injection equipment and procedures.
Safety
IPV is one of the safest vaccines in use. No serious systemic adverse reactions have been shown to follow vaccination.
Efficacy
IPV is highly effective in preventing paralytic disease caused by all three types of poliovirus.
Recommended use
An increasing number of industrialized, polio-free countries are using IPV as the vaccine of choice. This is because the risk of paralytic polio associated with continued routine use of OPV is deemed greater than the risk of imported wild virus.
However, as IPV does not stop transmission of the virus, OPV is used wherever a polio outbreak needs to be contained, even in countries which rely exclusively on IPV for their routine immunization programme.
Once polio has been eradicated, use of all OPV will need to be stopped to prevent re-establishment of transmission due to VDPVs.
The post Here is what you need to know about the two polio vaccines! appeared first on ARY NEWS.
ALSO VISIT FOR ONLINE EARNING Google Class





